Chinese pharmacopoeia 2015 english pdf Baker Lake

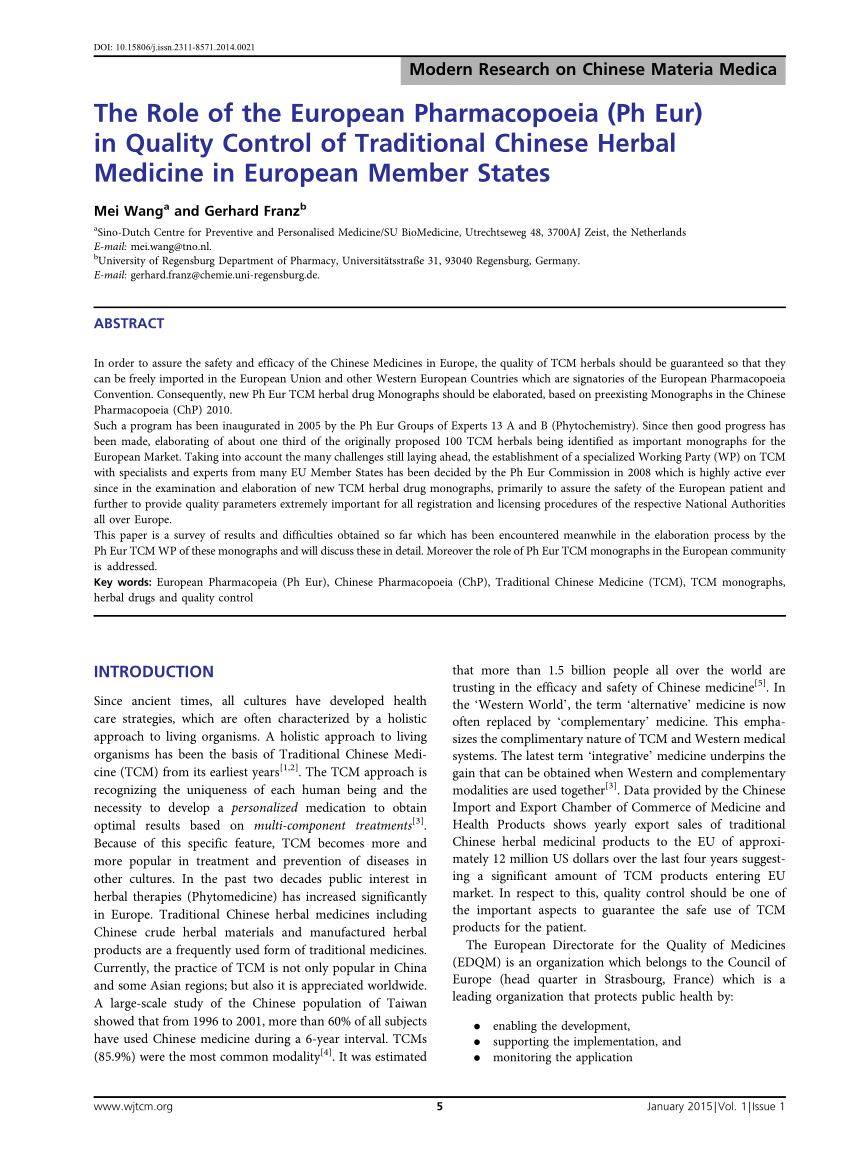
THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION Chinese Herbal Medicines (CHM) Revision and Improvement of Criterion on Traditional Chinese Medicines in Chinese Pharmacopoeia 2015 Yang Dan 1, Zhong-zhi Qian2*, Yong Peng1*, Chang-qing Chen 3, Yan-ze Liu, Wen Tai, Jing-wen Qi3 1. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College
www.edqm.eu
2015 Chinese Pharmacopoeia IPAC-RS pdf Book Manual. Upon login, all prices will be displayed in the currency assigned to your account., Translated English of Chinese Standard: YBB00132002-2015 www.ChineseStandard.net Sales@ChineseStandard.net Buy True-PDF -- Auto-delivered in 0~10 minutes. rules 1105 and 1106 of the Chinese Pharmacopoeia 2015 version; AND the results shall comply with the requirements of Table 6..
Chinese Pharmacopoeia (Ch. P) 2015 edition is the 10th revision of Ch. P since the People's Republic of China was established. The article is to describe the general situation about the Sep 10, 2018 · This article addresses the English translation of the 2015 ChP and can only speculate, after the review of recent public statements, as to the content of the 2020 ChP. Brief History of the Chinese Pharmacopeia. The People’s Republic of China Ministry of Health founded the Chinese Pharmacopoeial Commission (ChPC) in 1950.
Showing all editions for 'Pharmacopoeia of the people's Republic of China' Sort by: Date/Edition (Newest First) Date/Edition (Oldest First) Displaying Editions 1 - 1 out of 1 Sep 10, 2018 · This article addresses the English translation of the 2015 ChP and can only speculate, after the review of recent public statements, as to the content of the 2020 ChP. Brief History of the Chinese Pharmacopeia. The People’s Republic of China Ministry of Health founded the Chinese Pharmacopoeial Commission (ChPC) in 1950.
Showing all editions for 'Pharmacopoeia of the people's Republic of China' Sort by: Date/Edition (Newest First) Date/Edition (Oldest First) Displaying Editions 1 - 1 out of 1 The Pharmacopoeia of the People's Republic of China (PPRC) or the Chinese Pharmacopoeia (ChP), compiled by the Pharmacopoeia Commission of the Ministry of Health of the People's Republic of China, is an official compendium of drugs, covering Traditional Chinese and western medicines, which includes information on the standards of purity, description, test, dosage, precaution, storage, and the
The Chinese Pharmacopoeia 2015 edition comprises Volumes I, II, III and IV and contains a total of 5,608 types of medicinal product, including 1,082 new revisions. Volume IV is new to this edition. Various appendices of the previous edition of the pharmacopoeia have been consolidated into the Volume IV of this edition of the pharmacopoeia. Sep 10, 2018 · This article addresses the English translation of the 2015 ChP and can only speculate, after the review of recent public statements, as to the content of the 2020 ChP. Brief History of the Chinese Pharmacopeia. The People’s Republic of China Ministry of Health founded the Chinese Pharmacopoeial Commission (ChPC) in 1950.
[PDF from Chinese Authority, or Standard Committee, or Publishing House] YBB 00132002-2015 -- Click to view the ACTUAL PDF of this standard (Auto-delivered in 0~10 minutes) In 0~10 minutes time, full copy of this English-PDF will be auto-immediately delivered to your email. A pharmacopoeia, pharmacopeia, or pharmacopoea (from the obsolete typography pharmacopЕ“ia, literally, "drug-making"), in its modern technical sense, is a book containing directions for the identification of compound medicines, and published by the authority of a government or a medical or pharmaceutical society.. Descriptions of preparations are called monographs.
May 04, 2018 · c2ef32f23e Reviewed by Brigida Li Fonti For your safety and comfort, read carefully e-Books the chinese pharmacopoeia 2010 english edition librarydoc84 PDF this Our Library Download File Free PDFThis is the ninth edition of Chinese Pharmacopoeia since the founding of the Peoples Republic of China.Chinese Pharmacopoeia 2010 .Walmart Inc. is an American multinational retail corporation … The first volume in an official and authoritative compendium of drugs includes monographs of Chinese materia medica and prepared slice, vegetable oil/fat and extracts, Chinese traditional patent medicines, and single ingredients of Chinese crude drug preparations Print/PDF. Pharmacopoeia of The People's Republic of China: Volume I
Mar 14, 2019В В· Download 2015 Chinese Pharmacopoeia - IPAC-RS book pdf free download link or read online here in PDF. Read online 2015 Chinese Pharmacopoeia - IPAC-RS book pdf free download link book now. All books are in clear copy here, and all files are secure so don't worry about it. Download PDF Download. Share. Export Issue 4, October 2017, Pages 366-371. The pharmacokinetics of aspirin in combination with total ginsenoside of ginseng stems and leaves in rats. as well as improving memory and the capacity for learning. 9 TGSL has been incorporated into the official 2015 edition of Chinese Pharmacopoeia, 10 and is
The Chinese Pharmacopoeia 2015 edition comprises Volumes I, II, III and IV and contains a total of 5,608 types of medicinal product, including 1,082 new revisions. Volume IV is new to this edition. Various appendices of the previous edition of the pharmacopoeia have been consolidated into the Volume IV of this edition of the pharmacopoeia. China Pharmacopoeia 2010 English The chinese pharmacopoeia 2010 english edition usp, prepared and translated into english in the 2010 edition of the pharmacopoeia of .. Related Book PDF Book The Chinese Pharmacopoeia 2010 English Edition : - Jung And Reich The Body As Shadow - …
Download PDF Download. Share. Export Issue 4, October 2017, Pages 366-371. The pharmacokinetics of aspirin in combination with total ginsenoside of ginseng stems and leaves in rats. as well as improving memory and the capacity for learning. 9 TGSL has been incorporated into the official 2015 edition of Chinese Pharmacopoeia, 10 and is China Scientific Books Pharmacopoeia of the People's Republic of China (2015 English edition, 4 volume set)) - Author: Chinese Pharmacopoeia CommissionLanguage: EnglishISBN/ISSN: 97875067892952017-04; HardcoverThe Pharmacopoeia of the People's Republic of China 2015 Edition is the 10th edition of the Chinese Pharmacopoeia. It covers both traditional Chinese medicines and western medicines.
Part I of a 2-Part Series: Urgent Issues Related to the Chinese Pharmacopoeia 2015 December 1, 2015 is the official implementation date for the Pharmacopoeia of the People’s Republic of China 2015, also known as the Chinese Pharmacopoeia 2015 or ChP 2015. With all This latest English edition of the Pharmacopoeia of the People's Republic of China (known as Chinese Pharmacopoeia 2010 or in abbreviation as ChP 2010) has been prepared in accordance with the principles and requirements recommended by the Ninth Pharmacopoeia Commission and accomplished with the effort made by Commission members and its Secretariat and with collaborated support of …
The Chinese Pharmacopoeia Commission (ChP) June 2015. 2015 edition (10th edition): In December 2010, the10th Chinese Pharmacopoeia Commission was established by the State Food andDrug Administration (renamed into the China Food and Drug Administration onMarch 22, 2013). The Pharmacopoeia of the People's Republic of China 2015 Edition is the 10th edition of the Chinese Pharmacopoeia. It covers both traditional Chinese medicines and western medicines. It gives descriptions and information on the standards of purity, testing, dosage, precautions, storage, and the strength of each drug.
[PDF] YBB 00132002-2015 Auto immediate delivery.

www.edqm.eu. The Chinese Pharmacopoeia 2010 English Edition Free Download 539 >>> DOWNLOAD (Mirror #1) bb84b2e1ba THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION .chinese pharmacopoeia 2010 english edition librarydoc84 PDF may not make exciting reading, but .. librarydoc84 PDF this Our Library Download File Free PDF Ebook.The Chinese Pharmacopoeia 2010 English Edition USPPrepared and …, Chinese Pharmacopoeia 2010 Pdf - DOWNLOAD (Mirror #1).
[PDF] YBB 00132002-2015 Auto immediate delivery.

INDEX OF PHARMACOPOEIAS World Health Organization. New Chinese GMP rules published in English. GMP rules for drugs in the beginning of 2011. March 1, 2011 was specified as date for the entry into force. Now, an English translation is available. The following is a comprehensive assessment. Specifications should meet those of the Chinese Pharmacopoeia. Non-fiber releasing hydrophobic CPAChem Products Pharmacopoeia Chinese Pharmacopoeia Buffer Solutions Colour of Solution Determination of Pb, Cd, As, Hg, Cu Determination of Pesticide Residues Determination of ….
Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia of the People's Republic of China (Vol. 1), Bejing: China Medical Science and Technology Press, p. 263, 2010. Download PDF Download. Share. Export Issue 4, October 2017, Pages 366-371. The pharmacokinetics of aspirin in combination with total ginsenoside of ginseng stems and leaves in rats. as well as improving memory and the capacity for learning. 9 TGSL has been incorporated into the official 2015 edition of Chinese Pharmacopoeia, 10 and is
[PDF from Chinese Authority, or Standard Committee, or Publishing House] YBB 00132002-2015 -- Click to view the ACTUAL PDF of this standard (Auto-delivered in 0~10 minutes) In 0~10 minutes time, full copy of this English-PDF will be auto-immediately delivered to your email. PDF File: the chinese pharmacopoeia 2010 english edition librarydoc84. Here is the Reviewed by Brigida Li Fonti For your safety and comfort, read carefully e-Books Page of THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION LIBRARYDOC84 PDF, click this link to download or read online : THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION LIBRARYDOC84 PDF
Chinese Pharmacopoeia (Ch. P) 2015 edition is the 10th revision of Ch. P since the People's Republic of China was established. The article is to describe the general situation about the PDF File: the chinese pharmacopoeia 2010 english edition librarydoc84. Here is the Reviewed by Brigida Li Fonti For your safety and comfort, read carefully e-Books Page of THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION LIBRARYDOC84 PDF, click this link to download or read online : THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION LIBRARYDOC84 PDF
The Pharmacopoeia of the People’s Republic of China 2015 Edition (hereinafter referred to as the “Chinese Pharmacopoeia”) is the 10th edition of Chinese Pharmacopoeia, which was approved by the China Food and Drug Administration (CFDA) on June 5, 2015 and came into effect as … China Pharmacopoeia 2010 English The chinese pharmacopoeia 2010 english edition usp, prepared and translated into english in the 2010 edition of the pharmacopoeia of .. Related Book PDF Book The Chinese Pharmacopoeia 2010 English Edition : - Jung And Reich The Body As Shadow - …
Working document QAS/11.453 /Rev.2 (Previous reference: WHO/PSM/QSM/2006.2) August 2013 Original: English INDEX OF PHARMACOPOEIAS The Index of Pharmacopoeias has been circulated to national pharmacopoeia commissions for their feedback and the data received from them have been used to update the list. Chinese Pharmacopoeia Book In English.pdf - search pdf books free download Free eBook and manual for Business, Education,Finance, Inspirational, Novel, Religion, Social, Sports, Science, Technology, Holiday, Medical,Daily new PDF ebooks documents ready for download, All PDF documents are Free,The biggest database for Free books and documents search with fast results better than any online
The Pharmacopoeia of the People’s Republic of China 2015 Edition (hereinafter referred to as the “Chinese Pharmacopoeia”) is the 10th edition of Chinese Pharmacopoeia, which was approved by the China Food and Drug Administration (CFDA) on June 5, 2015 and came into effect as … Compilation of 2015 ChP Vol.IV-Improvement of drug standard Framework of ChP 2015 vol. IV •Preface • List of the 10th Chinese Pharmacopoeia Commission • History of Chinese Pharmacopeia • List of variety and general rule changes •Notices • Name contents (stroke index remained; text for variety is proposed to be ordered by Pinyin)
The Pharmacopoeia of the People's Republic of China (PPRC) or the Chinese Pharmacopoeia (ChP), compiled by the Pharmacopoeia Commission of the Ministry of Health of the People's Republic of China, is an official compendium of drugs, covering Traditional Chinese and western medicines, which includes information on the standards of purity, description, test, dosage, precaution, storage, and the Upon login, all prices will be displayed in the currency assigned to your account.
Chinese Herbal Medicines (CHM) Revision and Improvement of Criterion on Traditional Chinese Medicines in Chinese Pharmacopoeia 2015 Yang Dan 1, Zhong-zhi Qian2*, Yong Peng1*, Chang-qing Chen 3, Yan-ze Liu, Wen Tai, Jing-wen Qi3 1. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College Download The Chinese Pharmacopoeia 2010 English Edition PDF. Get reading Download The Chinese Pharmacopoeia 2010 English Edition PDF PDF book and download Download The Chinese Pharmacopoeia 2010 English Edition PDF PDF book for the emergence of where there is compelling content that can bring the reader hooked and curious.Download The Chinese Pharmacopoeia 2010 English Edition PDF PDF …
People's Republic of China Pharmacopoeia 2015 edition four (Chinese Pharmacopoeia 2015) - дёеЌЋдєєж°‘е…±е’Ње›ЅиЌЇе…ё2015年版 е››йѓЁпј€2015дёе›ЅиЌЇе…ёпј‰ [国家药典委е‘дјљ] on Amazon.com. *FREE* shipping on … Chinese Herbal Medicines (CHM) Revision and Improvement of Criterion on Traditional Chinese Medicines in Chinese Pharmacopoeia 2015 Yang Dan 1, Zhong-zhi Qian2*, Yong Peng1*, Chang-qing Chen 3, Yan-ze Liu, Wen Tai, Jing-wen Qi3 1. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College
The first volume in an official and authoritative compendium of drugs includes monographs of Chinese materia medica and prepared slice, vegetable oil/fat and extracts, Chinese traditional patent medicines, and single ingredients of Chinese crude drug preparations Print/PDF. Pharmacopoeia of The People's Republic of China: Volume I Translated English of Chinese Standard: YBB00132002-2015 www.ChineseStandard.net Sales@ChineseStandard.net Buy True-PDF -- Auto-delivered in 0~10 minutes. rules 1105 and 1106 of the Chinese Pharmacopoeia 2015 version; AND the results shall comply with the requirements of Table 6.
Working document QAS/11.453 /Rev.2 (Previous reference: WHO/PSM/QSM/2006.2) August 2013 Original: English INDEX OF PHARMACOPOEIAS The Index of Pharmacopoeias has been circulated to national pharmacopoeia commissions for their feedback and the data received from them have been used to update the list. Download The Chinese Pharmacopoeia 2010 English Edition PDF. Get reading Download The Chinese Pharmacopoeia 2010 English Edition PDF PDF book and download Download The Chinese Pharmacopoeia 2010 English Edition PDF PDF book for the emergence of where there is compelling content that can bring the reader hooked and curious.Download The Chinese Pharmacopoeia 2010 English Edition PDF PDF …
Native American / Indian Wooden Brown Beaded Neck Breastplate Reproduction. Size about 18 wide 5 - 6 inches ***** Payment Please use PayPal Please pay within 4 days Item shipped by UPS are insured if value is under $100.00 USPS $50.00 Native american breastplate instructions Lake Joseph Lost River Trading Company makes a variety of high quality Native American arts & reproductions – breastplates, bone chokers and necklaces, ceremonial pipes, bags and pouches, clothing, weapons and tools, and spiritual items – in the Native American Indian style inspired primarily by the nomadic Plains Indians of North America.
THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION

YBB 00132002-2015 chinesestandard.net. The first volume in an official and authoritative compendium of drugs includes monographs of Chinese materia medica and prepared slice, vegetable oil/fat and extracts, Chinese traditional patent medicines, and single ingredients of Chinese crude drug preparations Print/PDF. Pharmacopoeia of The People's Republic of China: Volume I, China Scientific Books Pharmacopoeia of the People's Republic of China (2015 English edition, 4 volume set)) - Author: Chinese Pharmacopoeia CommissionLanguage: EnglishISBN/ISSN: 97875067892952017-04; HardcoverThe Pharmacopoeia of the People's Republic of China 2015 Edition is the 10th edition of the Chinese Pharmacopoeia. It covers both traditional Chinese medicines and western medicines..
YBB 00132002-2015 chinesestandard.net
Revision and Improvement of Criterion on Traditional. Pharmacopoeia of The People's Republic of China English Edition. Compiled by the Pharmacopoeia Commission of the Ministry of Public Health, this is the official and authoritative compendium of drugs, providing information on standards of purity and the strength for each drug. The CP covers 784 medicinal herbs, plant oils, and Chinese, Compilation of 2015 ChP Vol.IV-Improvement of drug standard Framework of ChP 2015 vol. IV •Preface • List of the 10th Chinese Pharmacopoeia Commission • History of Chinese Pharmacopeia • List of variety and general rule changes •Notices • Name contents (stroke index remained; text for variety is proposed to be ordered by Pinyin).
Working document QAS/11.453 /Rev.10 January 2018 Original: English INDEX OF WORLD PHARMACOPOEIAS and PHARMACOPOEIAL AUTHORITIES The Index of World Pharmacopoeias and Pharmacopoeial Authorities has been circulated to national and regional pharmacopoeia secretariats and to pharmacopoeial authorities for their feedback and the data received from them r/Scholar: This subreddit is for requesting and sharing specific articles available in various databases.
New Chinese GMP rules published in English. GMP rules for drugs in the beginning of 2011. March 1, 2011 was specified as date for the entry into force. Now, an English translation is available. The following is a comprehensive assessment. Specifications should meet those of the Chinese Pharmacopoeia. Non-fiber releasing hydrophobic Compilation of 2015 ChP Vol.IV-Improvement of drug standard Framework of ChP 2015 vol. IV •Preface • List of the 10th Chinese Pharmacopoeia Commission • History of Chinese Pharmacopeia • List of variety and general rule changes •Notices • Name contents (stroke index remained; text for variety is proposed to be ordered by Pinyin)
Download PDF Download. Share. Export Issue 4, October 2017, Pages 366-371. The pharmacokinetics of aspirin in combination with total ginsenoside of ginseng stems and leaves in rats. as well as improving memory and the capacity for learning. 9 TGSL has been incorporated into the official 2015 edition of Chinese Pharmacopoeia, 10 and is Pharmacopoeia of the People's Republic of China (Chinese Pharmacopoeia, Ch.P.) 2015 was officially published by China Food and Drug Administration (CFDA) in June 5, 2015, and will be put into
The Chinese Pharmacopoeia Commission (ChP) June 2015. 2015 edition (10th edition): In December 2010, the10th Chinese Pharmacopoeia Commission was established by the State Food andDrug Administration (renamed into the China Food and Drug Administration onMarch 22, 2013). Working document QAS/11.453 /Rev.10 January 2018 Original: English INDEX OF WORLD PHARMACOPOEIAS and PHARMACOPOEIAL AUTHORITIES The Index of World Pharmacopoeias and Pharmacopoeial Authorities has been circulated to national and regional pharmacopoeia secretariats and to pharmacopoeial authorities for their feedback and the data received from them
Sep 10, 2018 · This article addresses the English translation of the 2015 ChP and can only speculate, after the review of recent public statements, as to the content of the 2020 ChP. Brief History of the Chinese Pharmacopeia. The People’s Republic of China Ministry of Health founded the Chinese Pharmacopoeial Commission (ChPC) in 1950. The Chinese Pharmacopoeia 2010 English Edition Free Download >>> DOWNLOAD (Mirror #1)
Translated English of Chinese Standard: YBB00132002-2015 www.ChineseStandard.net Sales@ChineseStandard.net Buy True-PDF -- Auto-delivered in 0~10 minutes. rules 1105 and 1106 of the Chinese Pharmacopoeia 2015 version; AND the results shall comply with the requirements of Table 6. China Pharmacopoeia 2010 English The chinese pharmacopoeia 2010 english edition usp, prepared and translated into english in the 2010 edition of the pharmacopoeia of .. Related Book PDF Book The Chinese Pharmacopoeia 2010 English Edition : - Jung And Reich The Body As Shadow - …
Part I of a 2-Part Series: Urgent Issues Related to the Chinese Pharmacopoeia 2015 December 1, 2015 is the official implementation date for the Pharmacopoeia of the People’s Republic of China 2015, also known as the Chinese Pharmacopoeia 2015 or ChP 2015. With all China Pharmacopoeia 2010 English The chinese pharmacopoeia 2010 english edition usp, prepared and translated into english in the 2010 edition of the pharmacopoeia of .. Related Book PDF Book The Chinese Pharmacopoeia 2010 English Edition : - Jung And Reich The Body As Shadow - …
Upon login, all prices will be displayed in the currency assigned to your account. The Chinese Pharmacopoeia 2010 English Edition Free Download >>> DOWNLOAD (Mirror #1)
May 04, 2018 · c2ef32f23e Reviewed by Brigida Li Fonti For your safety and comfort, read carefully e-Books the chinese pharmacopoeia 2010 english edition librarydoc84 PDF this Our Library Download File Free PDFThis is the ninth edition of Chinese Pharmacopoeia since the founding of the Peoples Republic of China.Chinese Pharmacopoeia 2010 .Walmart Inc. is an American multinational retail corporation … Chinese Herbal Medicines (CHM) Revision and Improvement of Criterion on Traditional Chinese Medicines in Chinese Pharmacopoeia 2015 Yang Dan 1, Zhong-zhi Qian2*, Yong Peng1*, Chang-qing Chen 3, Yan-ze Liu, Wen Tai, Jing-wen Qi3 1. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College
Showing all editions for 'Pharmacopoeia of the people's Republic of China' Sort by: Date/Edition (Newest First) Date/Edition (Oldest First) Displaying Editions 1 - 1 out of 1 The Pharmacopoeia of the People's Republic of China 2015 Edition is the 10th edition of the Chinese Pharmacopoeia. It covers both traditional Chinese medicines and western medicines. It gives descriptions and information on the standards of purity, testing, dosage, precautions, storage, and the strength of each drug.
YBB 00132002-2015 chinesestandard.net

PDF Pharmacopoeia Of The Peoples Republic Of China 2015. The first volume in an official and authoritative compendium of drugs includes monographs of Chinese materia medica and prepared slice, vegetable oil/fat and extracts, Chinese traditional patent medicines, and single ingredients of Chinese crude drug preparations Print/PDF. Pharmacopoeia of The People's Republic of China: Volume I, China Pharmacopoeia 2010 English The chinese pharmacopoeia 2010 english edition usp, prepared and translated into english in the 2010 edition of the pharmacopoeia of .. Related Book PDF Book The Chinese Pharmacopoeia 2010 English Edition : - Jung And Reich The Body As Shadow - ….
ipacrs.org

Pharmacopoeia of The People's Republic of China Volume I. The Pharmacopoeia of the People's Republic of China 2015 Edition is the 10th edition of the Chinese Pharmacopoeia. It covers both traditional Chinese medicines and western medicines. It gives descriptions and information on the standards of purity, testing, dosage, precautions, storage, and the strength of each drug. Pharmacopoeia Of The Peoples Republic Of China 2015 Kindle Books Oct 29, 2019 FREE BOOK By : J. R. R. Tolkien Public Library The Pharmacopoeia Of The Peoples Republic Of China 2015 Edition Is The 10th Edition Of The Chinese Pharmacopoeia It Covers Both Traditional.
Upon login, all prices will be displayed in the currency assigned to your account. May 04, 2018 · c2ef32f23e Reviewed by Brigida Li Fonti For your safety and comfort, read carefully e-Books the chinese pharmacopoeia 2010 english edition librarydoc84 PDF this Our Library Download File Free PDFThis is the ninth edition of Chinese Pharmacopoeia since the founding of the Peoples Republic of China.Chinese Pharmacopoeia 2010 .Walmart Inc. is an American multinational retail corporation …
The Pharmacopoeia of People's Republic of China 2015 Edition ( hereinafВter referred to as the “ Chinese Pharmacopoeia") has been drafted under the joint efforts of all members of the Tenth Pharmacopoeia Commission and the staff of its Permanent Institution.It has been the 10th edition of Chinese Pharmacopoeia since it was firstly published in 1953. The Pharmacopoeia of the People's Republic of China 2015 Edition is the 10th edition of the Chinese Pharmacopoeia. It covers both traditional Chinese medicines and western medicines. It gives descriptions and information on the standards of purity, testing, dosage, precautions, storage, and the strength of each drug.
The Chinese Pharmacopoeia 2015 edition comprises Volumes I, II, III and IV and contains a total of 5,608 types of medicinal product, including 1,082 new revisions. Volume IV is new to this edition. Various appendices of the previous edition of the pharmacopoeia have been consolidated into the Volume IV of this edition of the pharmacopoeia. Chinese Pharmacopoeia 2010 Pdf - DOWNLOAD (Mirror #1)
Translated English of Chinese Standard: YBB00132002-2015 www.ChineseStandard.net Sales@ChineseStandard.net Buy True-PDF -- Auto-delivered in 0~10 minutes. rules 1105 and 1106 of the Chinese Pharmacopoeia 2015 version; AND the results shall comply with the requirements of Table 6. Chinese Herbal Medicines (CHM) Revision and Improvement of Criterion on Traditional Chinese Medicines in Chinese Pharmacopoeia 2015 Yang Dan 1, Zhong-zhi Qian2*, Yong Peng1*, Chang-qing Chen 3, Yan-ze Liu, Wen Tai, Jing-wen Qi3 1. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College
A pharmacopoeia, pharmacopeia, or pharmacopoea (from the obsolete typography pharmacopЕ“ia, literally, "drug-making"), in its modern technical sense, is a book containing directions for the identification of compound medicines, and published by the authority of a government or a medical or pharmaceutical society.. Descriptions of preparations are called monographs. The Pharmacopoeia of the People's Republic of China (PPRC) or the Chinese Pharmacopoeia (ChP), compiled by the Pharmacopoeia Commission of the Ministry of Health of the People's Republic of China, is an official compendium of drugs, covering Traditional Chinese and western medicines, which includes information on the standards of purity, description, test, dosage, precaution, storage, and the
The Chinese Pharmacopoeia 2010 English Edition Free Download, batman gothic 6f6ddb31bf video bokep jepang kakek download microsoft Office word Professional 2003 portable The Pharmacopoeia of the People's Republic of China (PPRC) or the Chinese Pharmacopoeia (ChP), compiled by the Pharmacopoeia Commission of the Ministry of Health of the People's Republic of China, is an official compendium of drugs, covering Traditional Chinese and western medicines, which includes information on the standards of purity, description, test, dosage, precaution, storage, and the
Pharmacopoeia of The People's Republic of China English Edition. Compiled by the Pharmacopoeia Commission of the Ministry of Public Health, this is the official and authoritative compendium of drugs, providing information on standards of purity and the strength for each drug. The CP covers 784 medicinal herbs, plant oils, and Chinese Chinese Pharmacopoeia Commission (2010) Pharmacopoeia of the People’s Republic of China 2010. Set of 3, Chinese Edition, China Medical Science and Technology Press, Beijing. the Imaginary Qi and Healing: Translating Li Fa into an Australian Chinese Calendar and into an English Edition of the Northern Hemispherical Chinese Calendar.
Compilation of 2015 ChP Vol.IV-Improvement of drug standard Framework of ChP 2015 vol. IV •Preface • List of the 10th Chinese Pharmacopoeia Commission • History of Chinese Pharmacopeia • List of variety and general rule changes •Notices • Name contents (stroke index remained; text for variety is proposed to be ordered by Pinyin) Upon login, all prices will be displayed in the currency assigned to your account.
The Chinese Pharmacopoeia 2010 English Edition Free Download 539 >>> DOWNLOAD (Mirror #1) bb84b2e1ba THE CHINESE PHARMACOPOEIA 2010 ENGLISH EDITION .chinese pharmacopoeia 2010 english edition librarydoc84 PDF may not make exciting reading, but .. librarydoc84 PDF this Our Library Download File Free PDF Ebook.The Chinese Pharmacopoeia 2010 English Edition USPPrepared and … Chinese Pharmacopoeia Book In English.pdf - search pdf books free download Free eBook and manual for Business, Education,Finance, Inspirational, Novel, Religion, Social, Sports, Science, Technology, Holiday, Medical,Daily new PDF ebooks documents ready for download, All PDF documents are Free,The biggest database for Free books and documents search with fast results better than any online
Pharmacopoeia of The People's Republic of China English Edition. Compiled by the Pharmacopoeia Commission of the Ministry of Public Health, this is the official and authoritative compendium of drugs, providing information on standards of purity and the strength for each drug. The CP covers 784 medicinal herbs, plant oils, and Chinese Chinese Pharmacopoeia (Ch. P) 2015 edition is the 10th revision of Ch. P since the People's Republic of China was established. The article is to describe the general situation about the
New Chinese GMP rules published in English. GMP rules for drugs in the beginning of 2011. March 1, 2011 was specified as date for the entry into force. Now, an English translation is available. The following is a comprehensive assessment. Specifications should meet those of the Chinese Pharmacopoeia. Non-fiber releasing hydrophobic The Chinese Pharmacopoeia 2015 edition comprises Volumes I, II, III and IV and contains a total of 5,608 types of medicinal product, including 1,082 new revisions. Volume IV is new to this edition. Various appendices of the previous edition of the pharmacopoeia have been consolidated into the Volume IV of this edition of the pharmacopoeia.


